Empowering Your Research
Cell Line Engineering
Whether you’re identifying novel targets through high-throughput screening, validating primary screens, or exploring drug-gene interactions, our team of cell experts specializes in stable cell line generation and cell engineering for disease modeling, tailored to meet your research needs.
Explore our comprehensive stable cell line generation services.
Assay Cell Engineering services for stable cell line generation
- Single gene knockout
- Multiple gene knockout
- Whole gene deletion
- Point mutation knockin cell line
- Reporter knockin cell line
- Custom gene knockin cell line
- Primary Cell Isolation
- Cell Immortalization Service
- iPSC Generation Service
- iPSC Differentiation Service
- Constitutive overexpression cell line
- Inducible overexpression cell line
- Luciferase reporter cell line
- Fluorescent cell line
- Beta-galactosidase reporter cell line
Didn’t find what you’re looking for? Consult our technical experts for deeper insights into assay cell line engineering.
Expedite Your Assay Development
Streamlined workflow for Assay cell line Engineering
The delivery timeline for a stable pool or stable cell line depends on factors such as the cell line, growth characteristics, and culturing requirements. Our custom cell line development services can be completed in as little as 8 weeks.
01.
Strategy and Design
- Understand your project needs.
- Develop a tailored plan.
- Provide detailed proposal.
- Offer transparent pricing
02.
Vector Construction
- Guided design
- Gene synthesis
- Vector cloning
03.
Stable Cell Line Generation
- Host cell analysis
- Gene delivery (transfection, electroporation, lentiviral transduction)
- Stable cell pool
- Single clone generation (antibiotic selection, limiting dilution, flow cytometry sorting, etc.)
04.
Cell Line Validation / QC
- PCR/qPCR
- DNA sequencing
- Western blot (protein expression analysis)
- Immunofluorescence
- Flow cytometry
- Stability test
05.
Cell Line / Data Delivery
- 2 cryovials of stable cell pool with 10^6 cells per vial (We can send more vials upon request)
- Cell pool/clone generation report
- CoA
06.
Add-On Assays
- High-throughput screening (HTS)
- Cell viability and proliferation
- Reporter assays
- Functional assays
- Assay customization upon request
A Case Study on Assay Cell Line Engineering
Customer Requirements: Knockout and Overexpression Cell Line Generation
- To generate a stable cell line with targeted gene knockout.
- To overexpress the gene of interest and its mutant form in the CRISPR knockout cell line
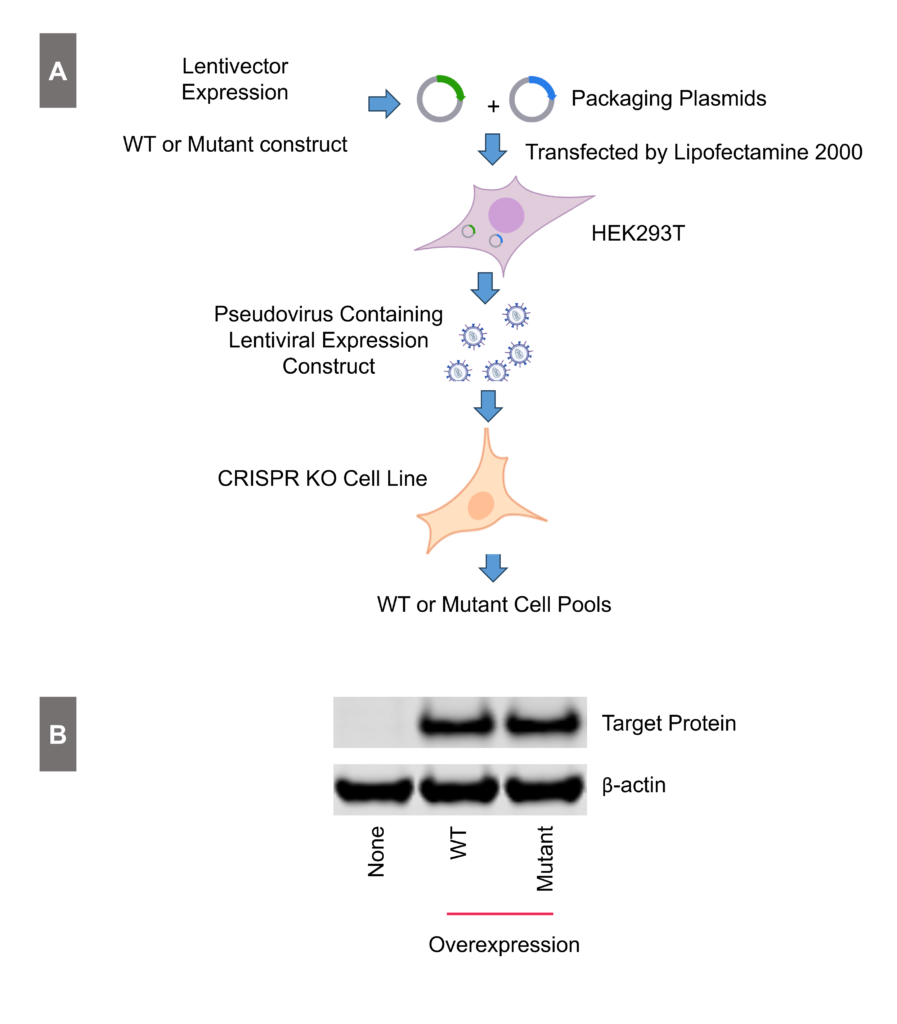
Overexpression Stable Cell Line Generation
Figure 2: Generation and validation of overexpression cell line. Introduce the expression of either WT and mutant target gene expression into the CRISPR knockout cell line. Panel A: Workflow for gene overexpression. Panel B: Validation of both WT and mutant overexpression using Western Blot analysis.
Generation of a CRISPR Knockout Cell Line
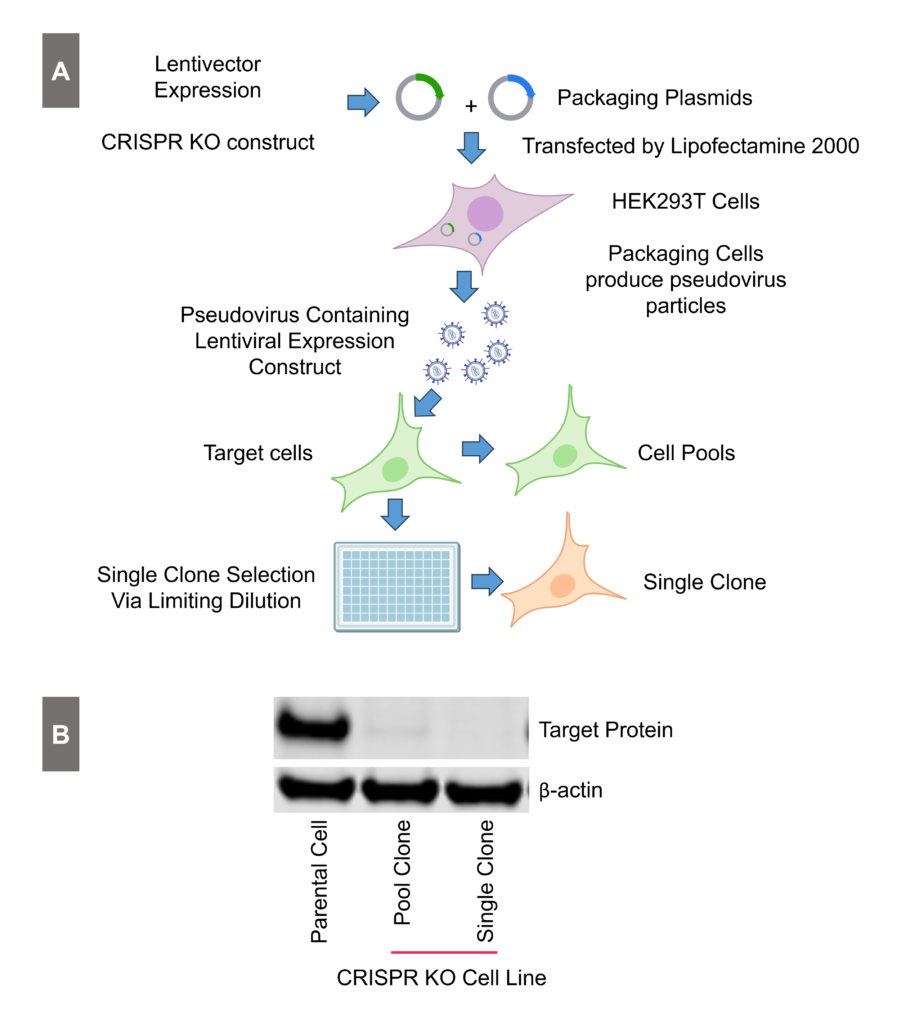
Figure 1: Generation and Validation of the CRISPR knockout cell line. To minimize the effects of endogenous target gene expression on small molecule compound screening. Panel A: Workflow for CRISPR gene knockout. Panel B: CRISPR knockout validation in both pooled and single clones using Western Blot analysis.
Please do not hesitate to contact us.
Visit our resource page to delve into our case studies, technical blogs, and ongoing promotions. Learn More

Related solutions
Cell-based Assays
Our cell-based assay development service leverages cell biology to craft robust assays, providing invaluable insights into hit/lead efficacy, toxicity, and mechanism of action for your research.
Frequently Asked Questions
We stand out from other CROs. Here, you’ll find answers to common inquiries regarding our company and stable cell line engineering services.
General Inquiry
San Diego, California.
Based in San Diego, California, we are a US-based company with a commitment to in-house operations for our custom stable cell line generation services. When you collaborate with us, rest assured that your projects are managed entirely within the United States, setting us apart from competitors who may outsource their services internationally.
We offer a comprehensive range of services tailored to meet your research and development needs. Our services include:
- Stable Cell Line Engineering: Customized generation of stable cell lines expressing your gene of interest using various techniques such as lentivirus transduction, CRISPR/Cas9 gene editing, or traditional transfection methods.
- Cell-Based Assay Development: Design and optimization of cell-based assays to evaluate compound efficacy, toxicity, and mechanism of action, including high-throughput screening assays, reporter gene assays, and functional assays.
We offer a multitude of advantages tailored to meet your needs.
- 50 Years of Experience: Providing solutions based on over five decades of collective industry experience.
- Cost Efficiency: Exceptional value coupled with unbeatable pricing in United States.
- Faster Data Delivery: Delivering stable cell lines in as few as 8 weeks, markedly exceeding the industry’s typical turnaround time.
- Confident Results: Implementing stringent quality control measures and validation protocols to ensure precise and reliable outcomes.
- 100% Customer Satisfaction: Your satisfaction is our top priority, and we guarantee responses within 24 hours to ensure your needs are promptly addressed and exceeded.
- US-based Company: Operating exclusively in the US, adhering to local regulations and standards, we eliminate international shipping for faster delivery, addressing concerns about lead times, cell line quality, and potential ethics issues.
Technology Platform
Gene editing, a form of genetic engineering, enables the introduction of permanent and site-specific genomic DNA modifications within the genome.
An array of molecular tools exists for controlling gene expression, encompassing genome editing nucleases, transposons, episomes, siRNA, and shRNA.
Notable examples of gene-editing nucleases utilized in research include meganucleases (MNs), zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)-associated endonucleases (Cas).
To direct the nuclease to the target site, MNs, ZFNs, and TALENs rely on protein-DNA interactions, whereas CRISPR-Cas systems are guided by RNA-DNA interactions, which provides several advantages over ZFNs and TALENs. These advantages include straightforward design for any genomic targets, easy prediction of off-target sites, and the ability to modify multiple genomic sites simultaneously (multiplexing).
CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is a revolutionary gene-editing technology derived from bacterial immune systems. It works by utilizing a guide RNA (gRNA) to direct a molecular scissors enzyme, typically Cas9, to a specific location in the genome. Once guided to the target site, the Cas9 enzyme makes a precise double stranded break in the DNA. This double stranded break is preferentially repaired in the cell by non-homologous end joining (NHEJ), a mechanism which frequently causes insertions or deletions (indels) in the DNA.
CRISPR technology offers unprecedented precision, efficiency, and versatility in genetic manipulation, making it a powerful tool for research, biotechnology, and potential therapeutic applications.
Transfection involves introducing nucleic acids into cells using non-viral methods, while transduction entails introducing foreign DNA into cells using viral vectors.
Lentiviral transduction is a process where genetic material is introduced into a target cell using a lentiviral vector. The lentiviral vector is engineered to carry the desired genetic payload, and once introduced into the target cell, it integrates the genetic material into the host genome. This integration enables stable, long-term expression of the introduced genes.
Lentivirus transduction is widely employed in genetic research, gene therapy, and stable cell line generation due to its ability to efficiently deliver and integrate genetic material into a broad range of cell types.
Get In Touch with us
